Science Safari: On the hunt for new medicines
We are currently living in a world where many of our known and trusted antibiotics are no longer effective against diseases they used to cure. With antibiotic resistance continuing to develop rapidly, the race is on to find new drugs to help patients.
In 1928 a bacteria-killing chemical was discovered in mould, and just over a decade later became the first antibiotic drug used to reliably treat infections in people - penicillin. Following on from this there began a boom in the discovery of new antibiotics in the 1950s, 60s and 70s, with scientists discovering new antibiotic compounds to turn into drugs all the time. Most of these antibiotics came from soil, which seemed like a hotbed of new medicines. After a while though, scientists began finding the same ones over and over again, and the Golden era of antibiotic discovery came to an end. The last new antibiotic was discovered in the 1980s, and scientists are realizing they must branch out and explore new, unique territories if they want to start finding new treatments again.
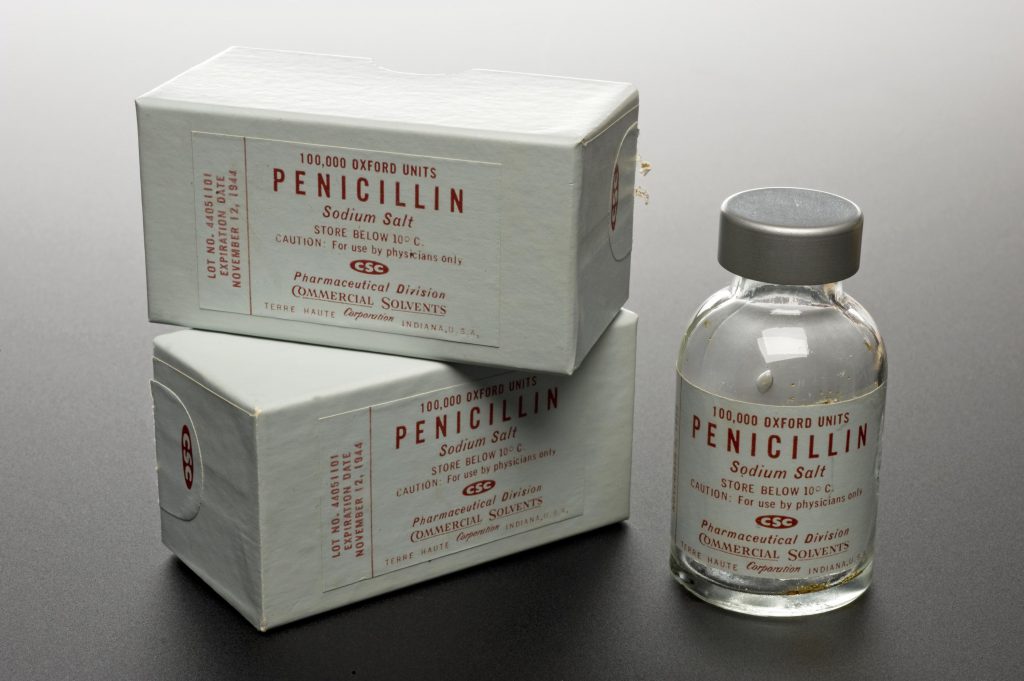
Image Credit: Science Museum Group Collection
Penicillin packages from 1944
Where are new antibiotics hiding?
The best place to look if you want to find undiscovered microbes with antibiotic properties are places with unique or unusual environments, those with specific conditions that are very different from other parts of the world. These might be areas with extreme hot or cold temperatures, places that are deep into the earth’s crust, or other places where the environment has led organisms to evolve in very specific ways. Wherever bacteria live there is competition, with individual species adapting to fight off others and become the dominant bacteria. If a bacterium is producing a chemical that kills off its neighbors, we might also be able to use that chemical to kill the bacteria that’s making us unwell. In these special geographies, we are more likely to find microbes that are producing chemicals we wouldn’t find in any other places. Unique and relatively unexplored areas where bacteria are left to their own devices to evolve these mechanisms without the influence of humans are therefore the best spots to search for potential new drugs.

Down to earth
Most of the current antibiotics that have come from nature were found by searching soil, but that doesn’t mean that there isn’t anything left for us to discover in the ground. Whilst the easily accessible areas have been searched over, there are many harder to reach or less well explored areas that could have secrets lurking in its soil. This is the basis of Rockefeller University’s ‘Drugs from Dirt’ initiative. The team want to find out all the chemicals produced by soil bacteria across the world and build a huge world map of these chemicals to help scientists develop new drugs. This would have been impossible to do as just one team, so they enlisted the public for help. In 2015 they asked people across the globe to collect some soil and send it off to their lab in New York to be analyzed. They made it clear that what they were looking for was not your average back garden dirt, but soil from unusual or remote locations.

This initiative seems to be doing the trick, as in 2018 the team announced the discovery of a new class of antibiotic from the soil. Whilst the journey from discovery to useable, licensed medicines is long, it’s a promising first step on the way to finding some new treatments for bacterial infections.
Into the depths
We know more about some parts of space than we do about our own oceans, which makes the deep sea a great place to start hunting for undiscovered bacteria. The deepest parts of the ocean, particularly that in the cold Arctic may seem empty and uninhabitable, but a surprising number of creatures do make these icy lands their home.
 https://pixabay.com/photos/glacier-lagoon-iceland-arctic-cold-498291/
https://pixabay.com/photos/glacier-lagoon-iceland-arctic-cold-498291/
PharmaSea is an EU funded project consisting of scientists from 13 countries who are sailing off on trips across the world to collect samples from the deepest, coldest and hottest places on the planet and investigate their bacteria-killing properties. One group from University of Tromsø, Norway head out to the Arctic sea twice a year for weeks at a time, gathering samples of water, the sea bed or creatures like sea sponges and bring them back to the lab to hunt for antibiotic properties. The team in Tromsø have already discovered some unique marine fungi that have antibacterial properties and they are now investigating them further to see if they could form the basis of future antibiotics.

A member of the team sorts the samples on an Arctic expedition in 2009
Image Credit: Marcel Jaspars
Ant-ibiotics
Sampling the environment is one way to find new potential antibiotics, but researchers at the John Innes Centre in Norwich, England are taking a different approach and using animals to help with their investigations. Ants are interesting creatures, with sophisticated social and infrastructure networks, and it turns out they’ve been using antibiotics for millions of years themselves. Some species of ants grow their own food by cultivating fungus gardens in underground tunnels. The most well-known of these ants are the Leafcutters, famous for their hard work breaking rainforest leaves down into tiny pieces and transporting them along trees back to their tunnels. The ants use these leaf segments to feed the fungus gardens and use their faeces as manure. To protect both their precious food supplies and the worker ants themselves against bacterial or fungal infections, the ants are inoculated with a strain of bacteria when they are born. These bacteria grow over their bodies as tiny white speckles, and exists in partnership with the ant, producing anti-bacterial and anti-fungal chemicals in return for food provided through special glands on the ants. These bacteria and fungi have been living and competing together for millions of years but haven’t developed resistance, so they are a great place for the team to explore for potential new drugs.

Leafcutter ants break down leaves into small pieces and carry them back to their underground homes
Usually, when sampling randomly in soil, only around one in a million samples contain a useful potential medicine. These ants are proving to be a valuable resource, as on contrast one promising strain of bacteria is found for every fifteen studied from an ant nest. In one ant colony the team found a previously undiscovered member of a bacterial family that seems to be effective against two types of superbug.
Under our noses
Bacteria are the most numerous organism on the planet, and have been found wherever people have looked for them – including inside our bodies. Living mainly in our gut and on our skin, we are home to more bacteria than human cells. Most of these bacteria are harmless, and some are even beneficial to us. Much like in soil and ants’ nests, the bacteria in our bodies are also competing with each other to be the most dominant. This means that our body’s native bacteria are producing chemicals to kill off other bacteria, which means our bodies could be another place to explore for new drugs. A research team at University of Tübingen in Germany decided to investigate the bacteria living inside our noses, as any bacteria that want to make a home in our nostrils will have to fight for the limited space available.
Their hunt was a success when they found S.lugdunensis, a strain of bacteria living in the nose that could put up a fight against S.aureus – a bug that usually lives harmlessly on our skin but can evolve into the antibiotic resistant superbug form MRSA that is responsible for thousands of deaths every year. The team found out exactly which part of the bacteria’s genetics were responsible for creating this S.aureus killing chemical, which they named lugdunen, and are hoping to use this to develop new drugs. They also surveyed the noses of 187 hospital patients, and found that the people who had these bacteria living in their noses had only one sixth of the dangerous S.aureus than those without. This is a promising finding which could suggest that as well as a treatment for MRSA, S.lugdunensis might be able to act as a probiotic as well – by introducing it into our noses we could create an ecosystem that prevents S.aureaus from moving into the neighbourhood of our noses.
Searching high and low for new antibiotics is a great way to make sure we have some treatments for infections that have become resistant, but bacteria will eventually grow resistant to the new ones too if we keep using antibiotics irresponsibly. Reducing the amount of antibiotics used and making sure they are used responsibly with prescriptions are also vital parts of solving this problem.
HOLLY PALMER
ASSISTANT CURATOR, EXHIBITIONS







